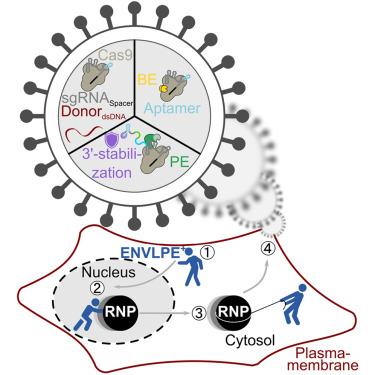
Graphic shows how the new system for gene editing can restore gene function in retinal disease models. The four-step process involves the engineered nucleocytosolic vehicles for loading of programmable editors (ENVLPE) system leveraging enhanced CRISPR ribonucleoproteim (RNP) loading and stabilization to efficiently deliver gene-editing effectors, such as nuclease, base and prime editors.
A research team funded by the National Eye Institute have developed a new virus-like particle (VLP) to enhance how specialists edit proteins causing inherited diseases.
This new VLP system, called engineered nucleocytosolic vehicles for loading of programmable editors — or ENVLPE — efficiently corrected two mutations associated with blindness. The researchers anticipate this CRISPR gene editing approach also being used to treat other inherited diseases, such as cystic fibrosis and familial hypercholesterolemia.
UC Irvine School of Medicine researchers, in collaboration with a team from the Helmholtz Munich research institute in Germany,
“We have previously demonstrated VLPs that can efficiently deliver CRISPR prime editors to restore sight in live mice,” said Samuel Du, an NEI research fellow working in the lab of Krzysztof Palczewski, Ph.D. at the University of California Irvine School of Medicine.
That previous work directly fused CRISPR components to VLP structural components. However, their partnering team at Helmholtz Munich research institute in Germany discovered an alternative mechanism for recruiting and packaging CRISPR prime editors into VLPs, overcoming several bottlenecks in the VLP production.
“Utilizing RNA-protein interactions, the VLPs underwent several rounds of modification and optimization to create highly efficient VLPs,” said Palczewski. “A key advantage of this system is that it is highly modular — any CRISPR system can easily be put into the ENVLPE VLPs with no complicated cloning and optimization of cleavage patterns like with other VLP systems.”
The team at Helmholtz Munich tested the ENVLPE VLPs in multiple CRISPR paradigms: gene knockout, homology-directed repair, base editing and CRISPR-activation assays. They also applied these VLPs in multiple cell types, including workhouse tissue culture lines, brain organoids and primary T cells.
The findings appear in the paper “Engineered nucleocytosolic vehicles for loading of programmable editors,” published in the May 2025 issue of Cell.
To read more, go to the University of California, Irvine news story