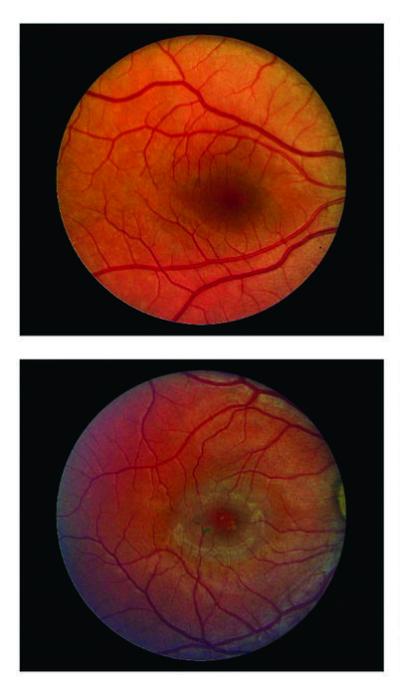
Images of a healthy retina (top) and a retina affected by macular telangiectasia type 2 (bottom). Credit: Scripps Research
For people with macular telangiectasia type 2 (MacTel), an orphan retinal disorder that gradually destroys central vision, there have long been no approved treatment options. But now, a new study spearheaded by investigators at Scripps Research and the National Eye Institute, part of the National Institutes of Health, offers compelling evidence that vision loss can be slowed with a neuroprotective surgical implant.
“This is a step toward redefining how we think about vision loss,” said one of the corresponding authors, Martin Friedlander, M.D., Ph.D., professor in the Department of Molecular and Cellular Biology at Scripps Research. “Instead of waiting for cells to die, we’re learning how to protect and preserve them.”
Published in in NEJM Evidence on July 22, 2025, the study reports results from two phase 3 clinical trials evaluating ENCELTO (revakinagene taroretcel-lwey), a surgically implanted device that continuously releases a therapeutic protein to preserve vision. Conducted across 47 sites internationally, the randomized trials enrolled 228 participants with MacTel, following their progress over a 24-month period.
The trials were coordinated by an international network of clinicians and researchers with key collaborators from the NIH’s National Eye Institute, the Lowy Medical Research Institute, and Neurotech Pharmaceuticals. Their ensuing results helped support the March 2025 approval of ENCELTO for MacTel by the US Food and Drug Administration (FDA), making it the first authorized treatment for the condition and the first cell-based neuroprotective treatment for any neurodegenerative retinal disease or central nervous system disorder.
The trials followed identical designs but enrolled participants at different times and locations. While both trials showed that ENCELTO slowed the loss of light-sensing retinal cells, the effect was stronger in one trial, likely due to variations in disease severity and other factors among participants. Together, the results provide strong evidence that the implant can preserve vision in people with MacTel.
“This is the first time we’ve seen a therapy meaningfully alter the course of MacTel,” said Friedlander. “It confirms that neuroprotection can be a powerful strategy to preserve vision in degenerative retinal conditions.”
Friedlander, who’s also president of the Lowy Medical Research Institute, has spent nearly two decades studying MacTel and helped lay the biological foundation for ENCELTO. The device delivers ciliary neurotrophic factor (CNTF), a naturally occurring protein known to protect retinal neurons. ENCELTO also consists of genetically modified retinal pigment epithelial cells—which help nourish and support the retina—housed in a tiny, collagen-based capsule, which is implanted in the back of the eye. Thanks to the capsule design, the cells remain shielded from immune rejection while continuously releasing CNTF—enabling long-term, localized delivery of the therapeutic molecule.
The study demonstrated that ENCELTO significantly slowed the loss of photoreceptors—light-sensing nerve cells that are critical for central vision—compared with sham-treated eyes, or eyes that underwent a simulated procedure without receiving actual treatment. In one of the trials, the implant resulted in a 54.8% reduction in the rate of ellipsoid zone loss, a measurable change in retinal structure that signals photoreceptor cell degeneration. The second trial showed a 30.6% reduction in the same measure, which is still statistically significant, though smaller in magnitude.
In addition to changes in retinal cells, the study evaluated several measures of visual function: how well the eye can actually see and perform everyday vision-related tasks. These included microperimetry (a sensitive test of retinal light response) and reading speed. Results from microperimetry showed statistically significant slowing of visual function loss, particularly in the trial where ENCELTO provided greater preservation of photoreceptor cells. However, reading speed and retinal sensitivity outcomes were more mixed, with one trial showing improvement and the other showing no significant difference from the control group.
“These differences highlight just how complex it is to measure functional vision loss in a slow-progressing disease like MacTel,” said Friedlander. “If you look at certain functional outcomes from just one of the trials, they're not statistically significant. But when you pool data from both trials—which were conducted the same way—then you see statistically significant results, so we’ll continue to investigate what’s driving that.”
Despite the variability, the overall trend across both trials supports ENCELTO’s long-term benefit, particularly when treatment is initiated before extensive cell loss. Participants tolerated the implant well, with minimal side effects. Furthermore, ENCELTO was effective regardless of the participant’s baseline vision or disease stage, suggesting that earlier intervention may help preserve more functional vision as MacTel progresses.
To read more, go to Scripps Research.