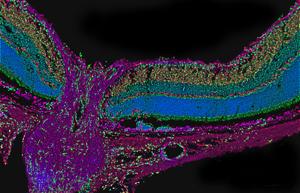
In cross-section, the optic nerve and retina take on a funnel shape. The optic nerve (the purple stem of the funnel) collects information from the retina (the mouth of the funnel) and sends it to the brain. Credit: Drs. Alejandra Bosco and Monica L. Vetter, University of Utah, Salt Lake City
Glaucoma is sometimes called the “silent thief of sight” because it slowly damages the eyes and can cause irreparable harm before there is any vision loss. But this disease is stealthy in more ways than one. Glaucoma has been known at least since antiquity, and yet, researchers today still do not know what causes it in most cases. There are treatments to delay vision loss, but no cure, making it a leading cause of blindness all over the world.
Glaucoma is a group of diseases that damage the optic nerve, a cable at the back of each eye that connects it to the brain. It affects more than 2.7 million people in the United States and more than 60 million worldwide. There are many forms of the disease, but primary open-angle glaucoma is the most common form and the most mysterious.

The eye is filled with fluid that drains through a gap between the cornea and iris. A build-up of fluid and eye pressure can damage the optic nerve.
High-pressure research
Since January is Glaucoma Awareness Month, it’s a good time to ask: Are researchers making progress in solving this mystery?
“Primary open-angle glaucoma remains a black box, but researchers are pursuing many avenues to investigate the underlying causes. As we develop a better understanding of the disease process, we hope this will lead to new, more effective treatments and possibly even preventive therapies for it,” said Hemin Chin, Ph.D., director of the Glaucoma and Optic Neuropathies program at the National Eye Institute (NEI), part of the National Institutes of Health.
If you’ve ever had an air-puff test, also known as tonometry, then you may have heard that glaucoma is linked to an increase in eye pressure, or intraocular pressure. The unique anatomy of the eye, when combined with other factors, can cause a rise in eye pressure that can in turn cause some types of glaucoma.
The front of the eye, between the cornea (the eye’s front window) and the iris (the colored part of the eye), is filled with a clear fluid. This fluid leaves the eye and enters the blood by passing through a gap at the angle where the cornea and iris meet. The gap is filled with a sponge-like tissue called the trabecular meshwork, which helps regulate fluid passage. Sometimes, eye infections, injuries, or certain medications can narrow the gap and compress this spongy tissue, producing a rapid buildup of fluid and eye pressure. This is called angle-closure glaucoma.
In the United States, open-angle glaucoma is more common than angle-closure glaucoma, affecting about three times as many people. It has a more gradual course and there are no clear signs of blockage within the eye’s drainage system. Yet, researchers estimate that 50 to 80 percent of people with open-angle glaucoma have eye pressure that is higher than average. Others have normal pressure or even low pressure. On the flip side, many people have high eye pressure but never develop glaucoma.
“Elevated intraocular pressure is a leading risk factor for primary open-angle glaucoma,” said Robert N. Weinreb, M.D., chair and distinguished professor of ophthalmology, and director of the Hamilton Glaucoma Center at the University of California, San Diego (UCSD). “The higher the intraocular pressure, the more likely the person is to develop glaucoma and the more likely it is to progress.”
Because of these data, medications that lower eye pressure are a mainstay of glaucoma treatment. In some cases, a doctor may recommend surgery to increase fluid drainage from the eye. Drugs, surgery, or both approaches together are often successful at slowing the course of open-angle glaucoma. The NEI-funded Ocular Hypertension Treatment Study also found that pressure-lowering eye drops can delay the onset of glaucoma in people with high eye pressure. But even with medication or surgery, open-angle glaucoma usually continues to attack the optic nerve and cause gradual vision loss.
Other clues to glaucoma
There are other risk factors for open-angle glaucoma besides high eye pressure, and they may provide some clues about what causes it. For example, age is a clear risk factor. Open-angle glaucoma is rare among Americans under age 50, but affects nearly eight percent of Americans over 80.
Other risks come into play long before we grow old and even before we’re born, including our ancestry. Open-angle glaucoma is about five times more common among African Americans and Mexican Americans compared to whites, and it has an earlier, more rapid course in African Americans. Family history also has a strong influence. A person’s risk of developing open-angle glaucoma is about 10 times higher if a parent or sibling has it, and the risk is higher still if an identical twin has it.
These data from family and twin studies have prompted researchers to dig more deeply into the genetics of open-angle glaucoma. It turns out that sometimes open-angle glaucoma is passed from parent to child due to defects within a single gene. Eight such genes have been identified so far, and they tend to cause glaucoma with an early onset, before age 50. Thanks to these discoveries, there are now genetic tests that can help people with early-onset glaucoma determine the risk that their children will inherit the disease.
Unfortunately, it’s rare that primary open-angle glaucoma can be traced to a single cause. In the vast majority of cases, experts theorize that the risk of the disease is influenced by many small genetic differences that vary from person to person. Alone, a single gene probably has a small impact on glaucoma risk.
“It’s like tossing little pebbles into a pool,” said Janey Wiggs, M.D., Ph.D., associate director of the Ocular Genomics Institute at the Massachusetts Eye and Ear Infirmary, part of Harvard Medical School in Boston. “They only have a big impact when a bunch of them are thrown in together.”

Dr. Janey Wiggs.
Credit: Massachusetts Eye and Ear Infirmary.
The search for glaucoma genes
Dr. Wiggs and other researchers are conducting ambitious studies to compare the full genetic makeup—or genome—of people who have open-angle glaucoma to people who are free from the disease. They hope to sort out all of the suspected genetic differences that put people at risk.
This research began more than 20 years ago. Two of Dr. Wiggs’ colleagues at Harvard—Susan Hankinson, Sc.D., and Louis Pasquale, M.D.—had begun monitoring for open-angle glaucoma cases and collecting genetic samples from participants in two large public health studies. Dr. Wiggs began another genetic study of open-angle glaucoma in 1996, based on patients seen through the glaucoma service at Massachusetts Eye and Ear. But progress in these studies was painstakingly slow.
The pace accelerated dramatically with the completion of the NIH-led Human Genome Project in 2003, and the advent of technology to rapidly and cheaply decode each person’s unique genome. Soon, Dr. Wiggs and her Harvard colleagues merged their efforts, and began working with researchers at other sites. In 2012, with funding and additional guidance from NEI, Dr. Wiggs began leading the NEI Glaucoma Human Genetics Collaboration (NEIGHBOR). This consortium includes 12 institutions across the U.S., and has collected genetic samples from about 4,500 patients and about 20,000 (control) individuals without the disease. Recently, it has been expanded to include a number of other ongoing studies, and to build a database of genetic and clinical information on glaucoma cases and controls, called the NEIGHBOR Heritable Overall Operational Database (NEIGHBORHOOD). There are also major genetic studies of glaucoma based in the United Kingdom, Australia, Singapore, and Iceland.
So far, these efforts have uncovered five regions of the genome that are strongly associated with primary open-angle glaucoma, Dr. Wiggs said. Within each region, investigators have found specific genes that, together, form a line-up of suspects believed to contribute to the disease. “Every gene has its own story, but most of them divide neatly into two groups,” she said. “There are some that impact eye pressure and others that impact the optic nerve.”
Remember the trabecular meshwork, the spongy tissue that regulates fluid flow out of the eye? At least two of the genes linked to higher eye pressure and higher glaucoma risk appear to affect the function of this tissue, Dr. Wiggs said.
Genes affecting the cornea may also have an effect on glaucoma risk, she said. Many past studies have found that people with thin corneas are more likely to develop open-angle glaucoma. The NEIGHBOR consortium has found genetic factors that are associated with both a decrease in corneal thickness and an increase in the risk of open-angle glaucoma.
Linking genes with other risk factors
Another effort that is yielding data on glaucoma risk is the African Descent and Glaucoma Evaluation Study (ADAGES). This NEI-funded study was launched in 2002, in order to seek explanations for the high prevalence and rapid course of primary open-angle glaucoma in African Americans. Dr. Weinreb is leading it in collaboration with his UCSD colleague Linda Zangwill, Ph.D., and with Jeffrey Liebmann, M.D., at New York University Langone Medical Center, and Christopher Girkin, M.D., at the University of Alabama at Birmingham.
Soon after the study began, the researchers found that, compared to whites, African Americans have thinner corneas and subtle abnormalities in the visual field that aren’t detected with standard diagnostic tests. (The visual field is the area of space a person can see at a given instant without moving the eyes.) These abnormalities may be an early sign of disease. Recently, the study has expanded to include a search for genetic factors that put African Americans at risk for open-angle glaucoma.
“By identifying genetic and non-genetic factors associated with glaucoma in this high-risk population, we hope to develop better means to diagnose it and detect progression, and improved drug targets that could benefit all patients,” Dr. Weinreb said.
There is likely much more to glaucoma risk than a person’s genes. Lifestyle, environment, and other factors are also believed to play a role, experts say. Various studies have examined the potential role of smoking, alcohol, caffeine, fat intake, and exercise in open-angle glaucoma, but so far, none of these has shown a strong relationship with the disease. In women, earlier age at menopause has been linked to a higher risk of the disease. In collaboration with Dr. Pasquale at Harvard, Dr. Wiggs has found that in women, but not men, open-angle glaucoma is associated with small variations in genes that regulate the reproductive hormone estrogen. These findings have led to speculation that estrogen replacement therapy may help protect older women from glaucoma, but research on this topic is inconclusive.
“As a community, there’s still a lot we don’t know about glaucoma,” Dr. Wiggs said. “However, we’ve been able to achieve some success by pooling resources and ideas. I think we will see more rapid progress in the next few years.”

Dr. Robert Weinreb. Credit: UCSD
Protecting yourself against glaucoma
Even though the risks of glaucoma aren’t fully understood, remembering these tips can help you protect yourself and your family against it:
- People at higher risk include African Americans age 40 and older, everyone over age 60, especially Mexican Americans, and those with a family history of the disease.
- Because glaucoma often has no symptoms in its early stages, people at higher risk should have a comprehensive dilated eye exam every one to two years.
- With early detection and treatment, it is possible to slow progression of the disease and preserve vision.
NEI has more information about how to reduce your risk of vision loss from glaucoma, including this fact sheet and video. The NEIGHBORHOOD study is funded by NEI grant EY022305 and ADAGES is funded by NEI grant EY023704.
References:
Wiggs JL et al. “Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma.” PLoS Genetics, April 2012, DOI: 10.1371/journal.pgen.1002654. Wiggs et al Pubmed
Sample PA et al. “The African Descent and Glaucoma Evaluation Study (ADAGES): Design and Baseline Data.” JAMA Ophthalmology, September 2009, DOI: 10.1001/archophthalmol.2009.187. Sample et al Pubmed
Pasquale LR and Kang JH. “Lifestyle, Nutrition and Glaucoma.” Journal of Glaucoma, August 2009, DOI: 10.1097/IJG.0b013e31818d3899. Pasquale and Kang Pubmed
NEI leads the federal government’s research on the visual system and eye diseases. NEI supports basic and clinical science programs that result in the development of sight-saving treatments. For more information, visit http://www.nei.nih.gov.
Contact:
National Eye Institute
(301) 496-5248
neinews@nei.nih.gov