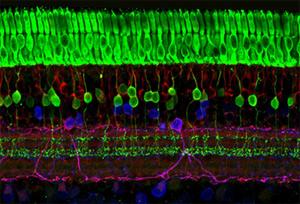
The retina has several layers of nerve cells. Photoreceptors (top, in green) are responsible for detecting light and converting it into electrical signals. Image courtesy of Wei Li, Ph.D., Unit on Retinal Neurophysiology, National Eye Institute.
Researchers have made progress toward an approach that would use light-sensitive drugs to stimulate cells in the retina and restore vision to people who are blind or visually impaired.
In retinitis pigmentosa (RP) and other diseases of the retina, vision loss is caused by a loss of photoreceptors. These are nerve cells that detect light and convert it into electrical signals, which are ultimately sent to the brain. The researchers have developed a set of light-sensitive compounds, called photoswitches, that could be used to bypass damaged photoreceptors.
The research is led by Richard Kramer, Ph.D., a professor of molecular biology and neurobiology at the University of California, Berkeley. In a recent study published in Neuron, Dr. Kramer and his colleagues describe a photoswitch that enables blind mice to respond to visible light. The study was funded in part by the National Eye Institute (part of the National Institutes of Health) and by the NIH Common Fund Nanomedicine initiative.
The broader focus of Dr. Kramer’s research is on ion channels, a key piece of machinery inside nerve cells that allows them to fire electrical signals. His research on photoswitches began in collaboration with Dirk Trauner, Ph.D., a professor of chemistry at Berkeley. About 10 years ago, the pair began developing photoswitches as a way to study and manipulate ion channels with light. They started with a light-sensitive chemical called azobenzene, and engineered new versions of it that could attach to ion channels and turn them on (or off) in response to light.
“It wasn’t a big jump from there to start researching photoswitches as a way to restore light sensitivity to the retina,” Dr. Kramer said.
Working with others at Berkeley and collaborators in Seattle and Munich, they found signs of promise with one of the first photoswitches they designed, called AAQ. In a 2012 Neuron paper, they reported that eye injections of AAQ restored some light sensitivity to mouse models of RP. As hoped, AAQ at least partly bypassed the need for photoreceptors and triggered firing by retinal ganglion cells in the optic nerve, which connects the retina to the brain.
But AAQ has properties that make it a poor candidate for drug development. For one, it is sensitive only to ultraviolet (UV) light, which is outside the spectrum of visible light seen by mammals. With prolonged exposure, UV light can also damage the retina. That makes AAQ impractical as a means to stimulate vision outside the lab. Moreover, in mice, AAQ was cleared from the eyes quickly, and its effects vanished within 12 hours of injection.
In the new study, Dr. Kramer and his colleagues performed similar tests with another derivative of azobenzene, called DENAQ. This molecule is sensitive to visible light, and it restored visible-light responses to RP mouse models.
The researchers first examined the effects of DENAQ on cellular responses in the retina. Similar to AAQ’s effects, DENAQ bypassed damaged photoreceptors and triggered firing by retinal ganglion cells. It also stimulated these cells directly, whereas AAQ is known to act on other retinal cell types, with more complex outcomes. DENAQ also had more persistent effects than AAQ, lasting up to several days after injection into the eye. Finally, while DENAQ enhanced retinal ganglion cell firing in mouse models of RP, it didn’t alter the cells’ firing rate in mice with healthy eyes. The researchers theorize that this could help reduce potential side effects from drugs based on DENAQ.
To determine if DENAQ helps restore sight in RP mouse models, the researchers examined behavioral responses in the mice. They watched how the mice behaved in light and darkness when they were placed in an unfamiliar space on their own, known as an open field test. When treated with DENAQ, the mice explored the space more actively with the lights on than in the dark. When left untreated, the mice were equally active in the light and dark.
But what can the mice see? “We know they can see light, but we can’t say we’ve restored their vision,” Dr. Kramer said. “We have to design further behavioral experiments to see if they can distinguish patterns and objects. We’re trying to do that now.”
Another goal is to better define how DENAQ triggers retinal ganglion cell responses, and to determine if the molecule can be fine-tuned even further, Dr. Kramer said. DENAQ appears to alter the activity of several types of ion channels within retinal ganglion cells, he said. But in the current study, he and his colleagues found evidence that DENAQ restores light sensitivity specifically by acting on a class of ion channels called HCN channels. Once DENAQ’s true target is found, the team hopes to once again design an improved photoswitch with even greater specificity and fewer potential side effects.
Dr. Kramer received support from NEI grants EY018957 and EY003176, and is a senior investigator with the NIH Nanomedicine Development Center for Optical Control of Biological Function (grant EY018241). Russell van Gelder, M.D., Ph.D., at the University of Washington in Seattle, is also a senior investigator with the center. Dr. van Gelder is leading efforts to move photoswitches from the lab to the clinic. For his proposal to treat retinal diseases with photoswitch drugs, he was one of ten winners in the Audacious Goals challenge, part of NEI’s larger Audacious Goals Initiative.
References:
Tochitsky, I et al. “Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells.” Neuron, February 2014. DOI: 10.1016/j.neuron.2014.01.003. Tochitsky et al Pubmed
Polosukhina, A et al. “Photochemical restoration of visual responses in blind mice.” Neuron, July 2012. DOI: 10.1016/j.neuron.2012.05.022. Polosukhina et al Pubmed